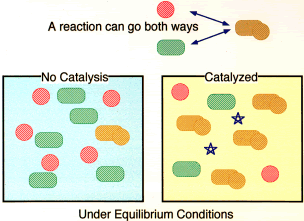
A binary chemical reaction can be seen as the combination of two elementary units into a single resulting compound. All such reactions can go both ways: the compound can split into the two elementary units. Under equilibrium conditions, there will be some of the compound and some of the components in the mix. How much there is of each will depend on many conditions, one of which is the presence and quantity of catalyst in the mix.
If a catalyst (stars in the right-hand diagrams) is added that enhances the likelihood that the components will associate to form the compound, there will be more of the compound and less of the components in the equilibrium mix. The more catalyst there is, the more of the compound and the less of the components there will be.
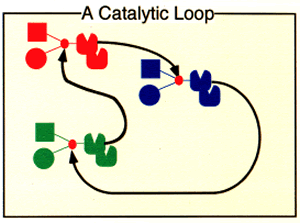
The product of one reaction can be a catalyst for a quite different reaction. The product of that reaction can catalyze another, and so forth. Eventually, one of the products may catalyze the original reaction, to complete a loop. If this happens, the loop is self-sustaining. The products of those reactions will increase at the expense of the products of any other reactions involving the same building block components.
It may be highly unlikely that the product of any specific reaction will catalyze any particular other reaction, but if there are enough components (and their reaction products) in the mix, it is almost certain that such a catalytic relationship will exist somewhere in the mix, and with more components and reaction products, at least one loop is almost certain to form.